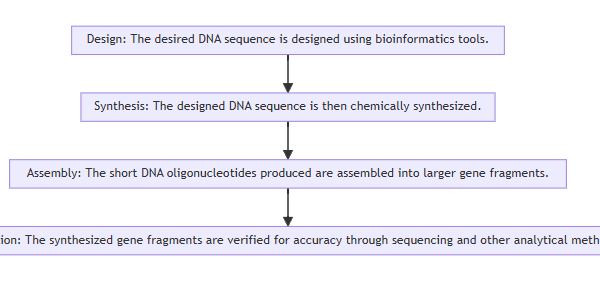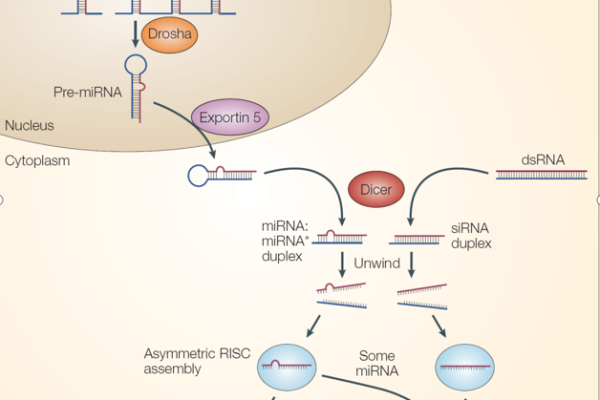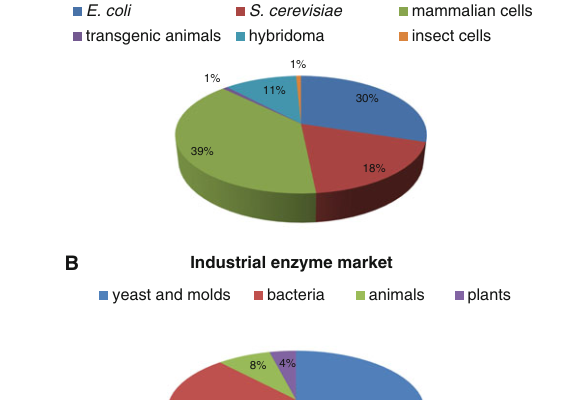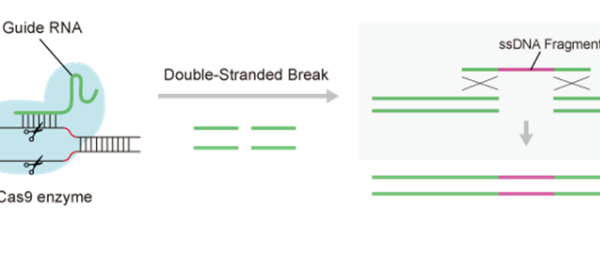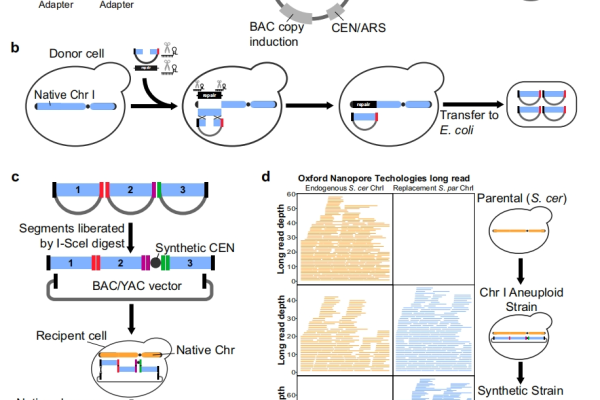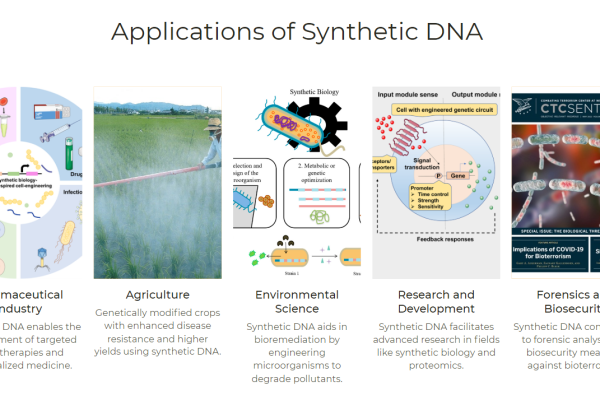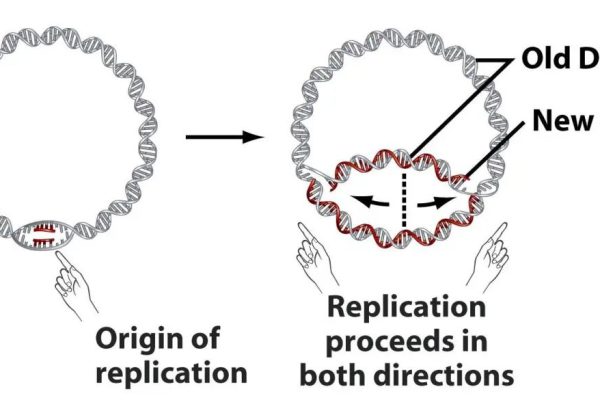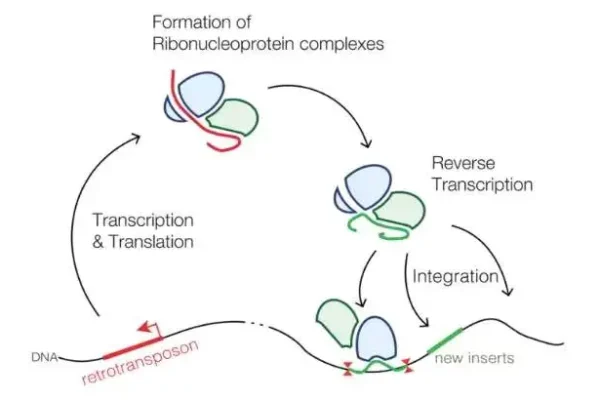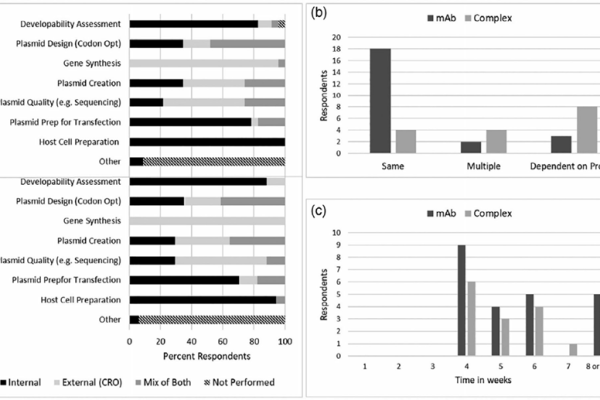
Detailed explanation of the development strategy for large factory cell lines
In the biopharmaceutical process, cell lines play a crucial role as a marker for the activation of CMC. A good cell line can not only reduce the difficulty of CMC development, but also greatly reduce the cost of drug development. However, the development of cell lines is a long and complex process, with multiple stages…