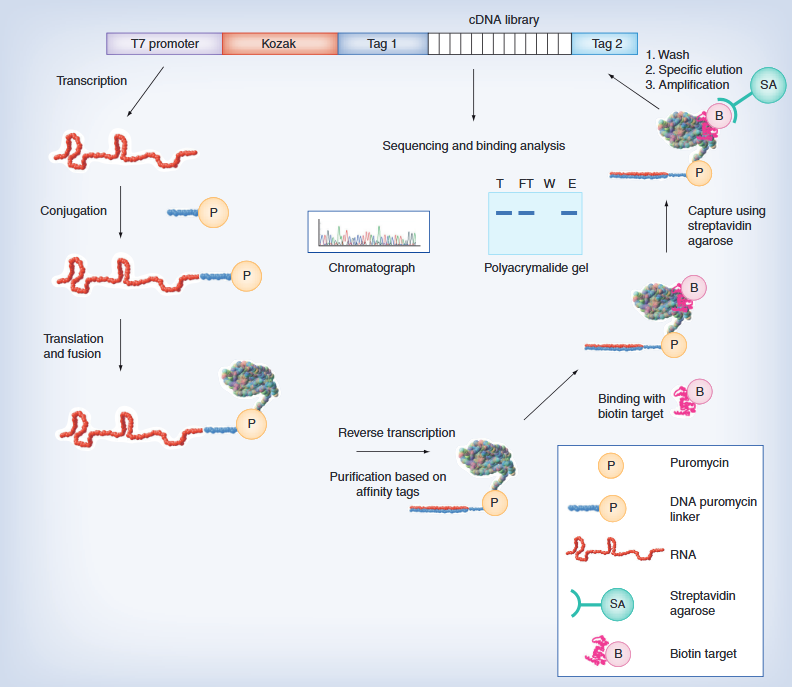
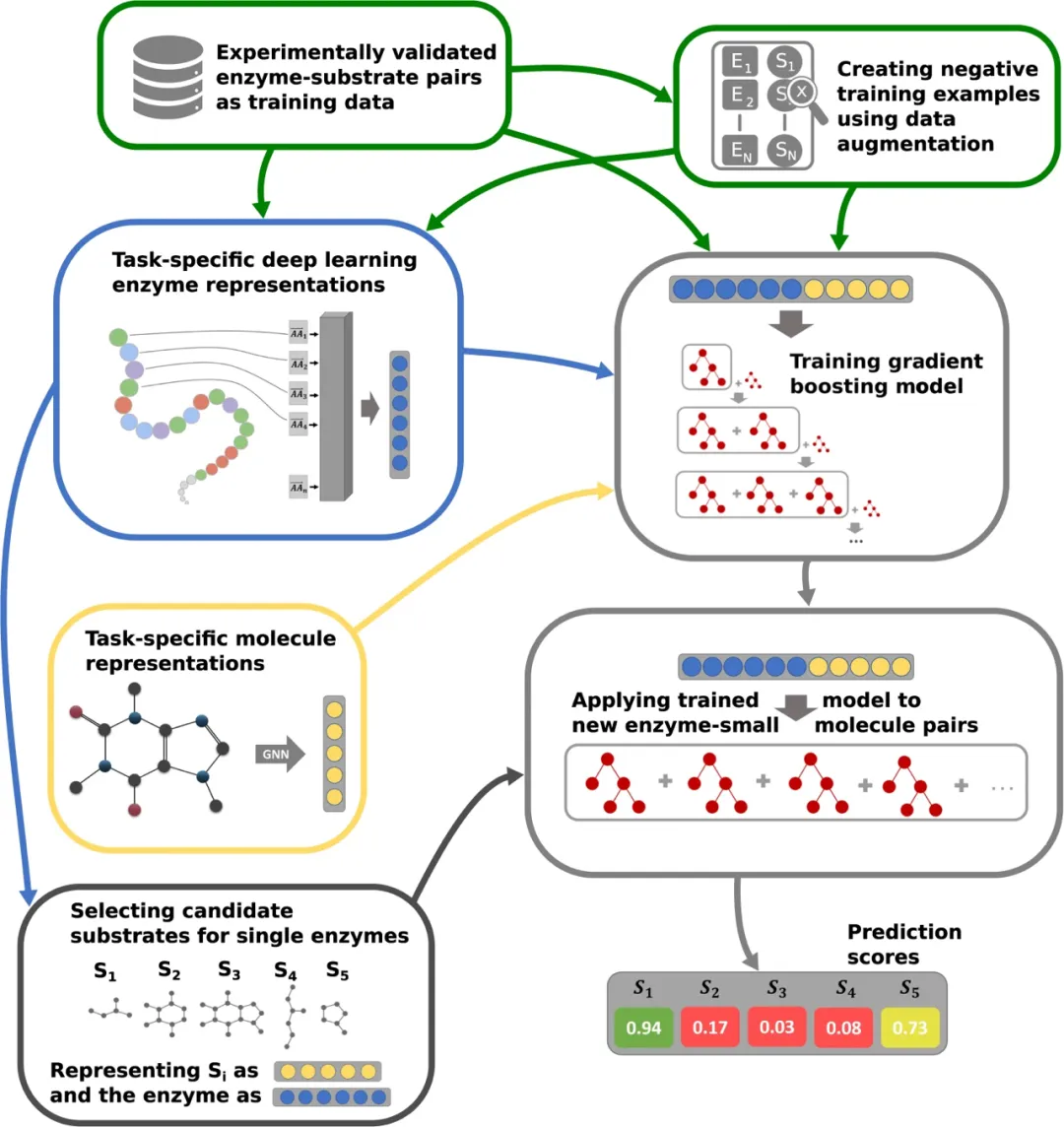
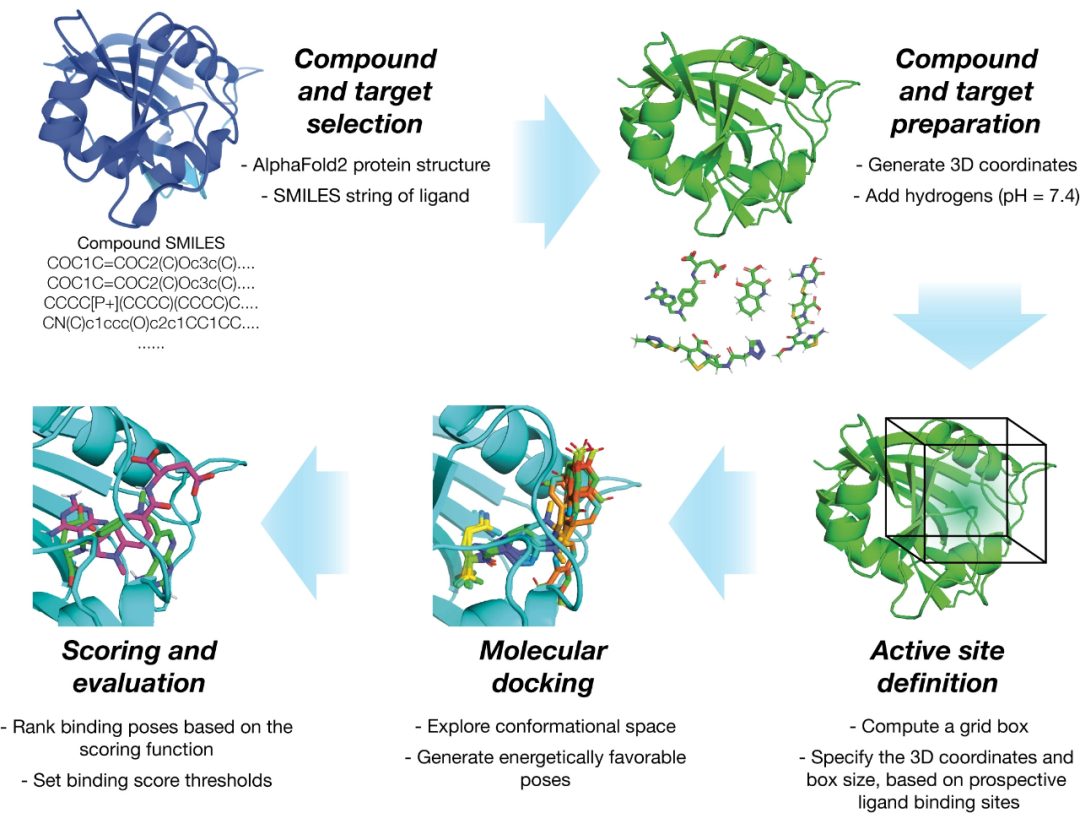
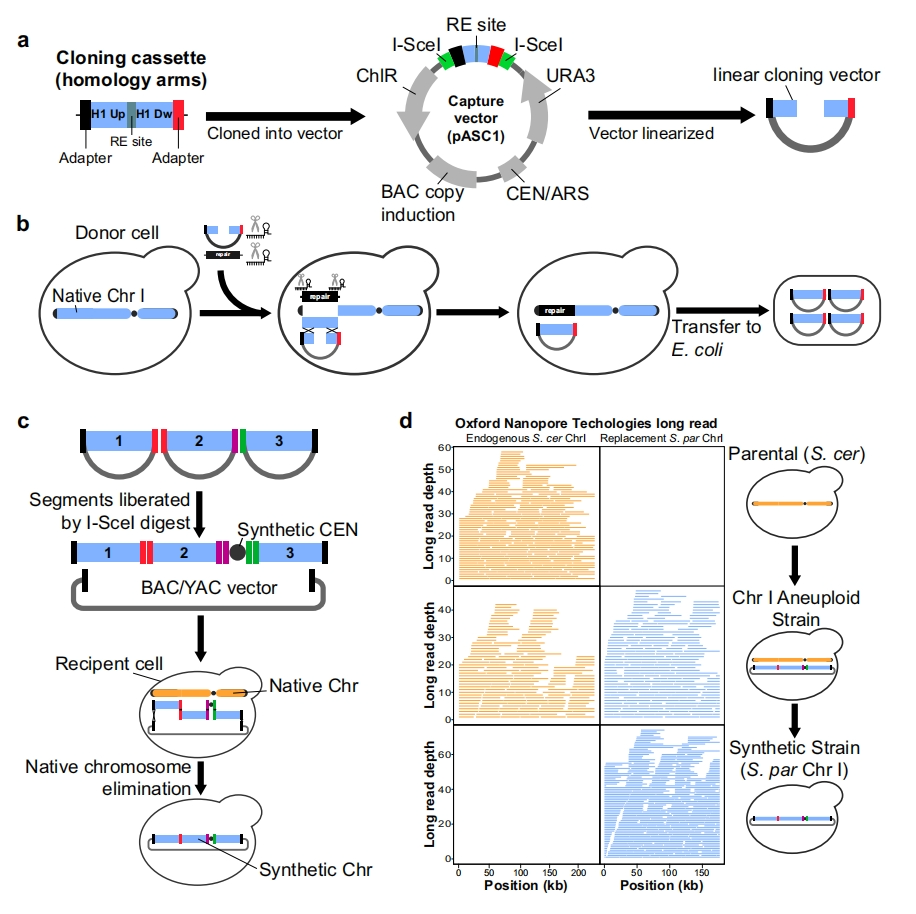
On November 16th local time in the United States, Vertex Pharmaceuticals (Nasdaq: VRTX) and CRISPR Therapeutics (Nasdaq: CRSP) announced that the UK Medicines and Healthcare Products Regulatory Authority (MHRA) has awarded their jointly developed CRISPR gene editing therapy CASGEVY ™ Conditional marketing license for exaagloggene autotemcel for the treatment of sickle cell anemia (SCD) and transfusion dependence β Thalassemia (TDT).

(Source: Company website)
It is understood that CASGEVY ™ It is a CRISPR gene editing therapy that includes artificial blood stem cells and progenitor cells edited in vitro through CRISPR-Cas9 in the erythroid specific enhancer region of the BCL11A gene.
There are two approved indications for this therapy in the UK. One is to treat sickle cell anemia patients aged 12 years and above who suffer from recurrent vascular occlusive crisis. These patients have β S/ β S, β S/ β+ Or β S/ β 0 genotype, suitable for hematopoietic stem cell transplantation and unable to obtain relevant hematopoietic stem cell donors matched with human leukocyte antigens; Another approach is to treat transfusion dependence for individuals aged 12 and above β Patients with thalassemia are suitable for hematopoietic stem cell transplantation and do not have relevant hematopoietic stem cell donors matched with human leukocyte antigens.
Sickle cell anemia is a hereditary blood disease that causes severe pain, organ damage, and shortened lifespan due to deformities or “sickle” blood cells. Patients experience painful vascular obstruction, also known as vascular occlusive crisis, which can lead to symptoms of acute chest syndrome, stroke, jaundice, and heart failure. In the UK, the average age of death for patients with sickle cell anemia is about 40 years old; β Thalassemia is also a hereditary blood disease, with a lack of red blood cells (anemia) being the main manifestation. Therefore, many people must undergo regular blood transfusions to meet the body’s needs. In the UK, β The average age of death for patients with thalassemia is about 55 years old. According to statistical data, approximately 2000 patients in the UK are eligible for CASGEVY ™ The applicable conditions for the therapy.
Today is a historic day in the history of science and medicine, and the UK is committed to CASGEVY ™ The authorization is the world’s first regulatory authorization for CRISPR based therapies According to Reshma Kewalramani, MD, CEO and President of Vertex Pharmaceuticals.
I hope this is the first time this Nobel laureate technology has been applied to eligible patients with severe illnesses, “said Dr. Samarth Kulkarni, Chairman and CEO of CRISPR Therapeutics.
According to official information, in CASGEVY ™ In two global clinical trials (CLIMB-11 and CLIMB-21) targeting SCD and TDT, these trials achieved their respective clinical endpoints of no severe recurrent vascular occlusion crisis or no dependence on blood transfusion for at least 12 consecutive months. Once these therapeutic effects are achieved, the benefits for patients may be lifelong.
So far, 97 out of these ongoing clinical trial studies have received CASGEVY ™ The safety of SCD and TDT patients treated is basically consistent with that of myeloablative therapy using Baixiaoan drugs and hematopoietic stem cell transplantation.
This authorization brings a new choice to eligible patients who are waiting for innovative therapies, and I look forward to patients receiving this treatment as soon as possible, “said Josu de la Fuente, the lead researcher of the clinical trials of CLIMB-11 and CLIMB-21 and the practical professor at Imperial College London.
It is worth noting that this is the first globally approved gene editing therapy based on CRISPR-Cas9. It is reported that the US FDA has approved the priority review of SCD and the standard review of TDT for this therapy, and has set the target action dates for the Prescription Drug Usage Fee Act (PDUFA) as December 8, 2023 and March 30, 2024, respectively.
Related recommendations
Genome Reprogramming
Genome Editing
CRISPR-based gene editing services
Base editing
Novel CRISPR system