Allosteric activation through mutational design is a strategy used in enzyme design to introduce or enhance the ability of an enzyme to be activated by specific molecules binding to allosteric sites. This approach involves introducing specific mutations in the enzyme's structure to create or improve allosteric communication between the allosteric site and the enzyme's active site, leading to activation.
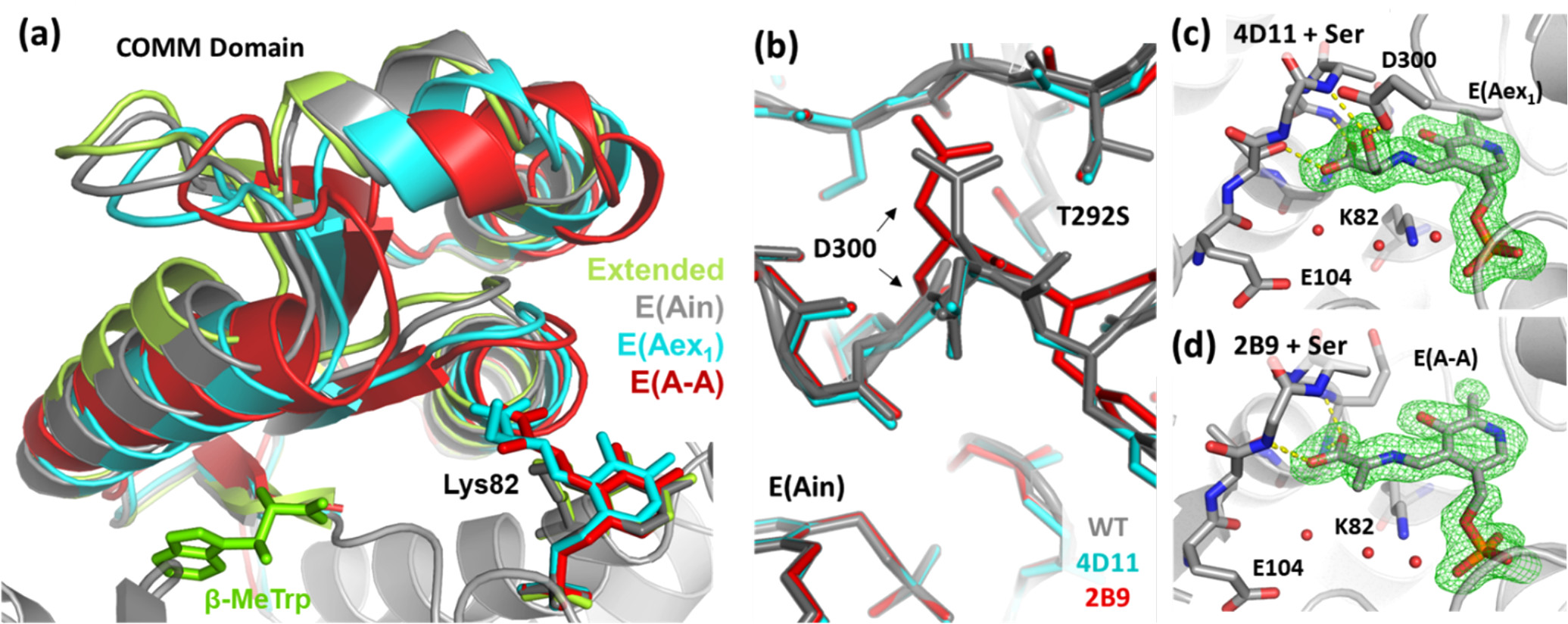 Fig. 1. Effects of Ser binding on the PfTrpB enzymes. (Buller, A.R.; et al., 2018)
Fig. 1. Effects of Ser binding on the PfTrpB enzymes. (Buller, A.R.; et al., 2018)
Our Process
- Allosteric Site Identification
The first step is to identify or create an allosteric site in the enzyme where the binding of a specific molecule can trigger activation. This can involve analyzing the enzyme's structure, using computational methods, or modifying the enzyme's active site or other regions to create a new allosteric site.
Once the allosteric site is identified, mutational analysis is performed to introduce specific mutations in the enzyme's structure. These mutations are strategically designed to enhance the communication between the allosteric site and the active site. The goal is to promote the transmission of allosteric signals upon the binding of the activating molecule.
- Computational Modeling and Simulation
Computational modeling and simulation techniques can be employed to predict the effects of the introduced mutations on the enzyme's structure and dynamics. Molecular dynamics simulations, for example, can provide insights into the conformational changes and allosteric communication pathways induced by the mutations.
Experimental characterization of the engineered enzyme is crucial to understand its activation mechanism. Kinetic assays can be performed to determine the activation constants, rate constants, and cooperativity associated with the allosteric activation process. This information helps in understanding the impact of the introduced mutations on the enzyme's activity and regulation.
- Iterative Design and Optimization
The mutational design process may involve iterative cycles of mutation, characterization, and optimization. Based on the experimental results and computational insights, further rounds of mutational design can be performed to fine-tune the enzyme's activation properties and improve its efficiency and specificity.
Advantages
In enzyme design, allosteric site design for substrate inhibition can be employed to engineer enzymes with enhanced regulatory properties and control over their activity. Here are some specific applications of allosteric site design for substrate inhibition in enzyme design:
- Specificity: Allosteric activation allows for specific regulation of enzyme activity by a particular molecule. Through mutational design, the enzyme can be engineered to respond only to the desired activating molecule, resulting in precise control over enzyme activity. This specificity is valuable in applications where targeted activation or regulation of enzymes is required.
- Orthogonality: Allosteric activation can be orthogonal to the enzyme's natural regulatory mechanisms. By introducing mutations to create an allosteric site, the activation process can be decoupled from the enzyme's endogenous regulatory pathways. This enables the activation of the enzyme independently of its natural modulators, expanding the range of control options available.
- Tunability: Mutational design allows for fine-tuning of the allosteric activation properties of the enzyme. By iteratively introducing mutations and characterizing the resulting enzyme variants, it becomes possible to optimize the activation constants, cooperativity, and other kinetic parameters. This tunability offers flexibility in tailoring the enzyme's activation response to meet specific requirements.
- Improved Catalytic Efficiency: Allosteric activation can enhance the catalytic efficiency of an enzyme. By activating the enzyme through an allosteric mechanism, it can reach its active conformation more readily, resulting in increased catalytic activity. This can be particularly beneficial in applications where high enzyme activity is desired, such as biocatalysis or metabolic engineering.
- Versatility: Allosteric activation through mutational design can be applied to a wide range of enzymes and enzyme families. The principles of allosteric communication and mutational design can be adapted to different enzyme structures and functions, making it a versatile strategy for enzyme engineering. This versatility opens up possibilities for the design of novel enzymatic functions across various fields.
- Potential for Multi-Input Control: Allosteric activation can enable the design of enzymes with multi-input control. By introducing multiple allosteric sites or engineering allosteric networks, it becomes possible to activate the enzyme through the binding of different molecules or combinations of molecules. This capability allows for more complex and sophisticated regulation of enzyme activity.
By choosing our services in allosteric activation through mutational design, you gain access to cutting-edge techniques, a team of experienced scientists, and a commitment to delivering high-quality results. We are dedicated to driving innovation in enzyme design and helping our clients unlock the full potential of their enzymes for various applications.
Reference
- Buller, A.R.; et al., Directed Evolution Mimics Allosteric Activation by Stepwise Tuning of the Conformational Ensemble. Journal of the American Chemical Society. 2018. 140(23): p. 7256-7266.

































 Fig. 1. Effects of Ser binding on the PfTrpB enzymes. (Buller, A.R.; et al., 2018)
Fig. 1. Effects of Ser binding on the PfTrpB enzymes. (Buller, A.R.; et al., 2018)