Allosteric site design for substrate inhibition involves modifying the allosteric properties of enzymes to introduce or enhance substrate inhibition as a regulatory mechanism. Substrate inhibition occurs when the binding of substrate molecules to the enzyme inhibits its own activity, resulting in a decrease in enzymatic reaction rates. We are pleased to offer cutting-edge services in allosteric site design for substrate inhibition in enzyme design.
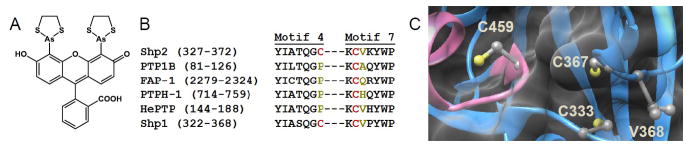 Fig. 1. Natural allosteric inhibitory site in PTP that could be used to engineer allosteric inhibitory sensitivity into PTP. (Reetz, M.T., et al., 2005)
Fig. 1. Natural allosteric inhibitory site in PTP that could be used to engineer allosteric inhibitory sensitivity into PTP. (Reetz, M.T., et al., 2005)
Our Services
- Allosteric Site Identification
The first step is to identify potential allosteric sites in the enzyme where the binding of specific molecules can lead to substrate inhibition. This can involve computational analysis, structural studies, and experimental characterization to identify regions that can modulate enzyme activity in response to substrate binding.
- Allosteric Modulator Screening
Once the allosteric site is identified, libraries of small molecules or compounds can be screened to identify potential allosteric modulators that induce substrate inhibition. High-throughput screening methods, virtual screening, or structure-based design approaches can be employed to identify compounds that bind to the allosteric site and trigger substrate inhibition.
Allosteric engineering techniques can be used to introduce or enhance substrate inhibition in the enzyme. This may involve site-directed mutagenesis, rational design strategies, or directed evolution to modify the allosteric site or its interactions with the enzyme's active site and substrate. The goal is to create an allosteric network that leads to substrate inhibition upon substrate binding.
- Kinetic Characterization and Modeling
Experimental determination of kinetic parameters associated with substrate inhibition, such as substrate inhibition constants, is crucial to understand the regulatory behavior of the engineered enzyme. Mathematical modeling and simulation can be performed to gain insights into the mechanism of substrate inhibition and predict the effects on enzyme activity under different conditions.
- Enzyme Optimization and Design
Based on the understanding of substrate inhibition and allosteric regulation, enzyme optimization and design strategies can be employed to fine-tune the regulatory properties. This can involve further engineering of the allosteric site, optimizing enzyme-substrate interactions, or incorporating additional regulatory elements to achieve the desired level of substrate inhibition.
Applications
In enzyme design, allosteric site design for substrate inhibition can be employed to engineer enzymes with enhanced regulatory properties and control over their activity. Here are some specific applications of allosteric site design for substrate inhibition in enzyme design:
- Fine-Tuning Enzyme Activity
Allosteric site design for substrate inhibition allows for precise control of enzyme activity. By introducing an allosteric site that induces substrate inhibition, it becomes possible to modulate the enzyme's catalytic efficiency and prevent excessive substrate turnover. This fine-tuning of enzyme activity is valuable in applications where precise control is required, such as biosensors or metabolic engineering.
- Metabolic Pathway Regulation
Allosteric site design for substrate inhibition can be utilized to regulate metabolic pathways. By incorporating enzymes with substrate inhibition properties, it becomes possible to control the flow of metabolites through the pathway. This regulation can prevent the accumulation of intermediates, balance metabolic fluxes, and optimize pathway efficiency.
Allosteric site design for substrate inhibition can be employed to introduce feedback inhibition in enzyme design. Feedback inhibition occurs when the end product of a metabolic pathway binds to the enzyme responsible for its production, leading to substrate inhibition and downregulation of the pathway. This regulatory mechanism can help maintain optimal levels of metabolites and prevent the wasteful overproduction of end products.
- Bioremediation and Detoxification
Allosteric site design for substrate inhibition has potential applications in bioremediation and detoxification processes. By engineering enzymes with substrate inhibition properties, it becomes possible to enhance their efficiency in degrading or detoxifying specific compounds. The substrate inhibition mechanism can prevent enzyme saturation and promote the efficient degradation of pollutants or toxins.
Allosteric site design for substrate inhibition can be utilized to engineer enzymes with new functions or altered substrate specificities. By introducing substrate inhibition in an enzyme, it becomes possible to modulate its affinity for different substrates. This can allow for the creation of enzymes with expanded substrate ranges or altered substrate preferences.
Reference
- Chio, CM.; et al. Rational design of allosteric-inhibition sites in classical protein tyrosine phosphatases. Bioorg Med Chem. 2015 Jun 15;23(12):2828-38.

































 Fig. 1. Natural allosteric inhibitory site in PTP that could be used to engineer allosteric inhibitory sensitivity into PTP. (Reetz, M.T., et al., 2005)
Fig. 1. Natural allosteric inhibitory site in PTP that could be used to engineer allosteric inhibitory sensitivity into PTP. (Reetz, M.T., et al., 2005)