CD Biosynsis is dedicated to provide our customers with best-in-class enzyme discovery and design services. We can expand the specificity of enzyme cofactors through our enzyme rational design service, where you can use enzymes for reactions that were not previously possible, or improve the efficiency and selectivity of enzyme reactions through the use of alternative cofactors.
Overview
Cofactors play a crucial role in catalytic activity, so altering the binding site of an enzyme cofactor is a powerful strategy for changing enzyme function. By manipulating cofactor binding sites, cofactors can influence enzyme specificity and activity, or even confer entirely new functions to the enzyme. Notably, modifying cofactor binding sites is a complex process that may require AI prediction and computer-aided design.
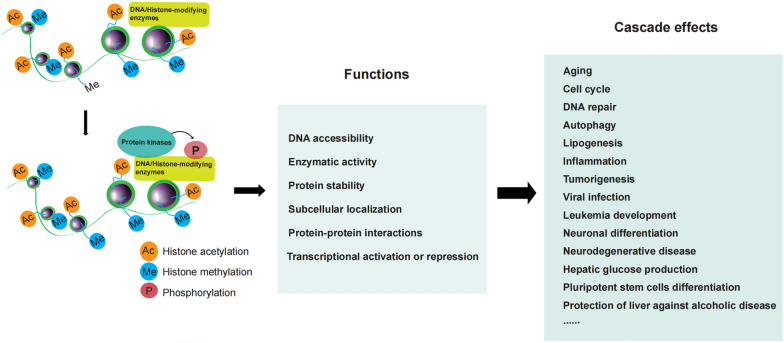 Fig 1. The model of transcriptional regulation of DNA/histone-modifying enzymes by phosphorylation. (Zhao P, et al., 2022)
Fig 1. The model of transcriptional regulation of DNA/histone-modifying enzymes by phosphorylation. (Zhao P, et al., 2022)
Our Services
Based on the research experience in enzyme design and evolution, our EnzymoGenius™ platform aims to provides enzyme cofactor binding site modification services, enabling researchers to better carry out enzyme engineering research and applications.
- Identify Cofactor Binding Residues
Firstly, we can obtain information about specific amino acid residues that are essential for cofactor binding from the crystal structure of the enzyme or using computerized prediction tools.
- Rational Design
Apply rational design principles to guide the modification of the cofactor site. Consider the chemistry of the native cofactor and the desired cofactor together and create a binding site designed to maintain catalytic efficiency while accommodating the altered cofactor.
- Site-Directed Mutagenesis
Use site-directed mutagenesis to introduce specific mutations in the identified cofactor binding residues. The general method is to replace the target amino acid with another amino acid in order to change the binding site.
- Screening and Selection
Develop high-throughput screening or selection methods to identify enzyme variants with desired cofactor binding properties. Screening methods mainly involve activity assays or other assays targeting altered function.
- Iterative Optimization
If needed, change the mutated residue sites based on the screening results and repeat the mutation and screening process.
- Validation of Altered Function
Validate whether the enzyme mutant exhibits the desired functional changes, including confirmation of changes in cofactor binding affinity, catalytic activity, and substrate specificity.
Our Advantages
- Professional Research Team
We have a professional research team of scientists with decades of experience in the field of enzyme design and evolution, focusing on the directed evolution and rational design of enzymes, and are committed to providing the greatest impetus for enzyme biology research.
- High-quality Services
We have a team of highly skilled and experienced scientists with in-depth knowledge of enzyme design evolution, who can provide you with professional consulting services before the project and reliable support services after the sale.
CD Biosynsis is a company that specializes in the rational design of enzyme cofactors to meet the specific research requirements of customers across various industries globally. Our expertise lies in expanding the specificity of these cofactors, allowing for enhanced enzymatic activity and improved performance in various applications. If you are interested in our services, please do not hesitate to contact us.
Reference
- Zhao, P.; Malik, S. The phosphorylation to acetylation/methylation cascade in transcriptional regulation: how kinases regulate transcriptional activities of DNA/histone-modifying enzymes. Cell Biosci. 2022,12(1):83.

































 Fig 1. The model of transcriptional regulation of DNA/histone-modifying enzymes by phosphorylation. (Zhao P, et al., 2022)
Fig 1. The model of transcriptional regulation of DNA/histone-modifying enzymes by phosphorylation. (Zhao P, et al., 2022)