EnzymoGenius™ pioneers custom substrate recognition through rational design, leveraging cutting-edge technologies to cater to diverse research needs. Our service is meticulously crafted to meet the evolving demands of enzymatic studies, offering bespoke solutions for enhanced substrate specificity.
Overview
Custom substrate recognition through rational design is a cutting-edge approach in the field of molecular biology, aiming to engineer biomolecules with tailored substrate specificity. This strategy involves the rational manipulation of molecular structures, leveraging insights from protein-ligand interactions and enzymatic mechanisms. Recent research progress in this domain has demonstrated significant advancements in the design of biomolecular recognition elements with enhanced selectivity for specific substrates. Employing principles of structural biology and computational modeling, scientists have successfully engineered proteins and nucleic acids capable of recognizing and interacting with custom substrates, opening avenues for applications in targeted drug delivery, biosensing, and synthetic biology. This innovative methodology holds promise for addressing challenges in various biological and biomedical contexts, offering a versatile platform for the development of precision tools and therapeutic interventions.
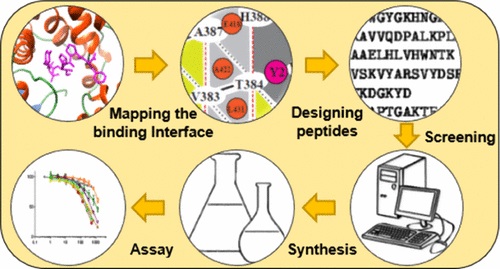 Fig. 1 Rational design of novel peptide ligands based on Knob–Socket-based for improved recognition of specific binding. (Uddin M Z, et al., 2019)
Fig. 1 Rational design of novel peptide ligands based on Knob–Socket-based for improved recognition of specific binding. (Uddin M Z, et al., 2019)
Our Services
- Tailored Substrate Design
Crafted substrates to meet specific enzymatic requirements, ensuring optimal performance in experimental setups.
- Computational Modeling
Utilizing advanced algorithms for molecular modeling, predicting substrate-enzyme interactions with precision.
- Biochemical Assay Development
Design and optimization of robust assays to validate enzymatic activity and substrate specificity.
- Structural Analysis
Employing structural biology techniques to elucidate the three-dimensional substrate-enzyme binding architecture, aiding in rational design.
- Kinetic Characterization
In-depth analysis of enzymatic kinetics to comprehend the substrate-enzyme interaction kinetics, facilitating accurate enzymatic studies.
Leading Technologies
- AI-Driven Design
Leveraging artificial intelligence for substrate design, enabling rapid and accurate prediction of potential interactions.
- High-Throughput Screening
Implementing state-of-the-art screening methods to expedite the identification of optimal substrates.
- Protein Engineering Expertise
Utilizing advanced protein engineering techniques to modify enzyme specificity and enhance substrate recognition.
- Comprehensive Database Integration
Incorporating extensive biochemical databases for informed substrate design, enhancing the rational design process.
Application Areas We Can Serve
- Enzyme Engineering
Tailoring enzymes for improved substrate specificity to meet industrial and biomedical applications.
- Drug Development
Designing substrates relevant to drug targets, aiding in the development of novel pharmaceutical compounds.
- Biocatalysis
Enhancing enzyme performance for sustainable biocatalytic processes in industrial applications.
- Metabolic Engineering
Designing substrates for enzymes involved in metabolic pathways for improved production of biofuels and chemicals.
- Diagnostic Assay Optimization
Tailoring substrates for diagnostic assays, ensuring high specificity and sensitivity in detection methodologies.
EnzymoGenius™ provides tailored solutions through advanced computational modeling, biochemical assay development, and structural analysis. Our AI-driven design, high-throughput screening, and protein engineering expertise set us apart, ensuring accurate and efficient substrate-enzyme interaction studies. With a focus on enzyme engineering, drug development, biocatalysis, metabolic engineering, and diagnostic assay optimization, our services cater to diverse research needs. Contact us today to elevate your enzymatic studies and accelerate scientific breakthroughs.
Reference
- Uddin, M. Z.; et al. Rational design of peptide ligands based on knob–socket protein packing model using cd13 as a prototype receptor. ACS Omega. 2019, 4(3): 5126-5136.

































 Fig. 1 Rational design of novel peptide ligands based on Knob–Socket-based for improved recognition of specific binding. (Uddin M Z, et al., 2019)
Fig. 1 Rational design of novel peptide ligands based on Knob–Socket-based for improved recognition of specific binding. (Uddin M Z, et al., 2019)