CD Biosynsis is an AI-led enzyme discovery and design company dedicated to providing customers with the best-in-class enzyme discovery and design services. Through our expanding enzyme cofactor specificity through rational design services, you can use enzymes in reactions that were previously impossible or increase the efficiency and selectivity of enzyme reactions through the use of alternative cofactors.
Overview
Enzymes are typically highly specific for their cofactors, which are molecules that are required for their activity. These cofactors can be in the form of metal ions, organic molecules, or vitamins. Through rational design, scientists can make targeted modifications to the enzyme's active site or binding pocket to allow the enzyme to accommodate different cofactors. The rational design approach may involve introducing specific mutations that alter the shape, charge, or interactions within the active site to match the desired cofactor. Additionally, rational design can involve introducing new binding sites or modifying existing binding sites to accommodate the new cofactor.
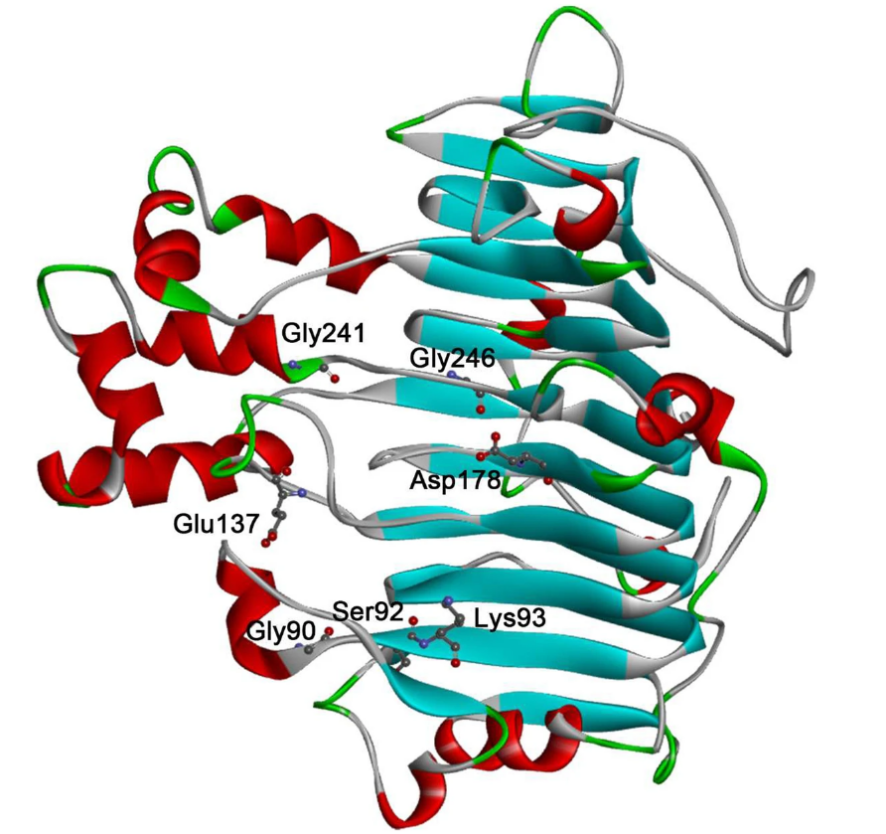 Fig. 1. The selected residues for possible stability enhancement by rational design. (Mak WS, et al., 2015)
Fig. 1. The selected residues for possible stability enhancement by rational design. (Mak WS, et al., 2015)
Our Services
Our rational design services to expand enzyme cofactor specificity involve modifying the active site of an enzyme to accept alternative cofactors or altering the cofactor binding region to accommodate different cofactor molecules.
- Cofactor analysis: Our experts work closely with you to analyze the structure and function of the cofactor of interest and determine the potential benefits or advantages it may have relative to natural cofactors to improve the catalytic capacity, stability, and availability of the target enzyme.
- Analyze active site and cofactor binding region: Determine the structure of the active site and binding region where cofactors interact with the enzyme. Identification of key amino acid residues involved in cofactor binding and their interactions with native cofactors.
- Identify conserved residues: Study homologous enzymes that naturally utilize the cofactor you wish to introduce and identify conserved amino acid residues involved in cofactor binding. These conserved residues often play important roles in cofactor recognition and can serve as potential targets for rational design.
- Assessing active site and cofactor regulation: Analyze structural compatibility between the active site and the new cofactor to identify potential conflicts or steric obstacles that may prevent successful binding of the new cofactor.
- Structural modeling and computational analysis: Use molecular docking or computational algorithms to predict the likely binding modes of new cofactors in enzyme active sites, and predicted binding affinities and energies were evaluated to narrow down the range of potential variants.
- Rational mutagenesis: Based on computational analysis, site-directed mutations are introduced into the amino acid residues in the active site and cofactor binding region to optimize the interaction between the enzyme and its substrate or cofactor.
- Enzyme characterization: After introducing mutations, our scientists experimentally characterize the activity and binding affinity of the enzyme to evaluate the effectiveness of the rational mutagenesis approach. Furthermore, through the repeated application of rational mutagenesis and experimental characterization, the interactions of enzymes with their substrates or cofactors can be fine-tuned, thereby improving catalytic activity and specificity.
CD Biosynsis is a company that specializes in the rational design of enzyme cofactors to meet the specific research requirements of customers across various industries globally. Our expertise lies in expanding the specificity of these cofactors, allowing for enhanced enzymatic activity and improved performance in various applications.If you are interested in our services, please contact us today
Reference
- Zhou Z.; et al. Rational design and structure-based engineering of alkaline pectate lyase from Paenibacillus sp. 0602 to improve thermostability. BMC Biotechnol. 2021, 21: 32.

































 Fig. 1. The selected residues for possible stability enhancement by rational design. (Mak WS, et al., 2015)
Fig. 1. The selected residues for possible stability enhancement by rational design. (Mak WS, et al., 2015)