EnzymoGenius™ stands at the forefront of rational protein-ligand interaction design for inhibition, offering a cutting-edge service that transcends conventional approaches. Our service employs a multifaceted strategy, combining computational methodologies and molecular modeling to provide tailored solutions for inhibiting specific protein targets with utmost precision.
Overview
The rational design of protein-ligand interactions for inhibition is a pivotal approach in drug discovery. It involves the systematic manipulation of molecular structures to optimize the binding affinity between a protein target and its ligand, aiming to modulate biological activities. Recent research progress in this field encompasses advanced computational methods, such as molecular docking and dynamics simulations, facilitating the identification of key binding interactions and energetics. Additionally, experimental techniques like X-ray crystallography and NMR spectroscopy contribute valuable structural insights. The integration of these approaches has led to the development of more effective and specific inhibitors, advancing our understanding of structure-activity relationships and enhancing the precision of drug design in the context of protein-ligand interactions.
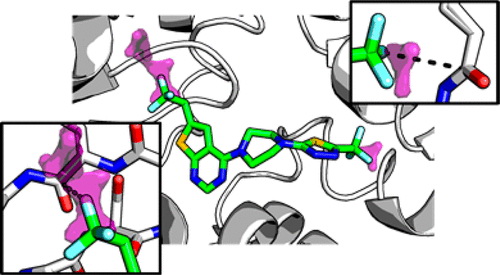 Fig. 1 Rational design of orthogonal multipolar interactions with fluorine in protein ligands. (Pollock J. W, et al., 2015)
Fig. 1 Rational design of orthogonal multipolar interactions with fluorine in protein ligands. (Pollock J. W, et al., 2015)
Our Services
- Computational Docking Analysis
Leveraging advanced algorithms, we conduct comprehensive computational docking analyses to predict the binding modes and affinities of ligands with the target protein.
- Binding Free Energy Calculations
Our service includes meticulous calculations of binding free energy, ensuring a quantitative understanding of the thermodynamics governing protein-ligand interactions.
- Structural Bioinformatics
Employing state-of-the-art structural bioinformatics tools, we identify key residues and structural motifs critical for ligand binding, facilitating the rational design of potent inhibitors.
Application Areas We Can Serve
- Drug Discovery
EnzymoGenius™ specializes in the rational design of protein-ligand interactions to expedite drug discovery processes.
- Enzyme Inhibition Studies
Our service caters to researchers and pharmaceutical companies conducting studies on inhibiting specific enzymes implicated in various diseases.
- Biological Pathway Modulation
We offer expertise in designing ligands that modulate biological pathways by selectively targeting key proteins.
- Structure-Based Drug Design
Our service is integral to structure-based drug design efforts, providing valuable insights for optimizing lead compounds.
Why Choose Us?
- High Precision Modeling
Our approach goes beyond standard methodologies, incorporating high-precision modeling techniques that enhance the accuracy of predicting protein-ligand interactions.
- Customized Design Strategies
Unlike one-size-fits-all approaches, EnzymoGenius™ tailors design strategies based on the unique characteristics of each target protein, optimizing the potential for successful inhibition.
- Integration of Experimental Data
We integrate experimental data into our computational models, ensuring a more realistic representation of the biological system and enhancing the reliability of our predictions.
EnzymoGenius™ commits to precision, customization, and integration of experimental data sets us apart in the realm of protein-ligand interaction design. With applications spanning drug discovery, enzyme inhibition studies, and pathway modulation, our services cater to a diverse range of scientific endeavors. We invite researchers and pharmaceutical professionals seeking innovative and tailored solutions to contact us.
Reference
- Pollock, J. W.; et al. Rational design of orthogonal multipolar interactions with fluorine in protein–ligand complexes. Journal of Medicinal Chemistry. 2015, 58(18): 7465-7474.

































 Fig. 1 Rational design of orthogonal multipolar interactions with fluorine in protein ligands. (Pollock J. W, et al., 2015)
Fig. 1 Rational design of orthogonal multipolar interactions with fluorine in protein ligands. (Pollock J. W, et al., 2015)