Overview
Tailoring enzyme active sites for covalent catalysis involves modifying the active site of an enzyme to promote and enhance covalent interactions between the enzyme and its substrate during catalysis. Covalent catalysis is a mechanism by which enzymes form transient covalent bonds with the substrate, facilitating the chemical transformation of the substrate into a product.
In covalent catalysis, the active site of the enzyme contains functional groups that can undergo covalent interactions with the substrate. These interactions can stabilize reaction intermediates, lower the activation energy of the reaction, and enhance the rate of the catalytic process. By tailoring the active site of an enzyme, scientists can optimize its ability to perform covalent catalysis, thereby improving its catalytic efficiency and specificity.
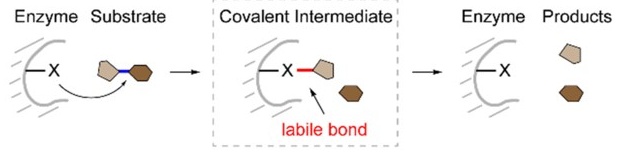 Fig. 1. Covalent catalysis process. (Wang & Tang, 2023)
Fig. 1. Covalent catalysis process. (Wang & Tang, 2023)
Advantages
Offers new possibilities for enzymatic catalysis
- Expansion of substrate scope
- Versatility in reaction conditions
Enhanced Catalytic Efficiency
- Accelerate the catalytic process by lowering the activation energy of the reaction
- Increased reaction specificity
- Control over reaction kinetics
Applications
- Enzyme Inhibitors: Covalent catalysis can be utilized to design enzyme inhibitors that irreversibly bind to the enzyme's active site, blocking its activity. By tailoring the active site to form a covalent bond with the inhibitor, the inhibitor achieves a prolonged and potent inhibitory effect. This approach is particularly valuable in drug discovery and development, where irreversible inhibition can enhance the efficacy and duration of action of therapeutic agents.
- Enzyme Activation: Covalent catalysis can be employed to activate enzymes by modifying their active sites. By introducing functional groups or reactive moieties in the active site, enzymes can be activated through covalent bonding with specific substrates or cofactors. This strategy allows for precise control over enzyme activity and can be applied in various fields, including biocatalysis and metabolic engineering.
- Substrate Specificity Engineering: Tailoring enzyme active sites for covalent catalysis enables the design of enzymes with altered substrate specificity. By introducing specific modifications in the active site, enzymes can be engineered to form covalent bonds with substrates that they would not typically interact with. This approach expands the range of substrates that an enzyme can accept, facilitating the development of enzymatic processes for the synthesis of valuable compounds.
- Catalytic Antibodies: Covalent catalysis can be employed in the design of catalytic antibodies, also known as abzymes. By modifying the active sites of antibodies, they can be engineered to possess enzymatic activity and catalyze specific reactions through covalent interactions with substrates. This approach has applications in antibody-based therapies, diagnostics, and chemical biology.
- Bioconjugation and Bioorthogonal Chemistry: Tailoring enzyme active sites for covalent catalysis can be utilized in bioconjugation reactions and bioorthogonal chemistry. Enzymes with modified active sites can be engineered to selectively react with specific functional groups or tags, enabling site-specific labeling, protein conjugation, or bioorthogonal click chemistry reactions. This approach is valuable in various bioanalytical and biotechnological applications.
- Protein Engineering: Covalent catalysis strategies can be employed in protein engineering to introduce or enhance enzymatic activities in non-enzymatic proteins. By modifying the active sites of non-enzymes to enable covalent interactions with substrates, these proteins can acquire catalytic capabilities and perform specific reactions. This expands the range of enzymatic functions available for various applications.
Our company is pleased to assist you in ordering our service for tailoring enzyme active sites for covalent catalysis. To proceed, please contact us. Our team will promptly reach out to you to finalize your order.
Reference
- Wang, Y.; Tang, S. Capturing Covalent Catalytic Intermediates by Enzyme Mutants: Recent Advances in Methodologies and Applications. ChemBioChem. 2023. 24(10), e202300036.

































 Fig. 1. Covalent catalysis process. (Wang & Tang, 2023)
Fig. 1. Covalent catalysis process. (Wang & Tang, 2023)