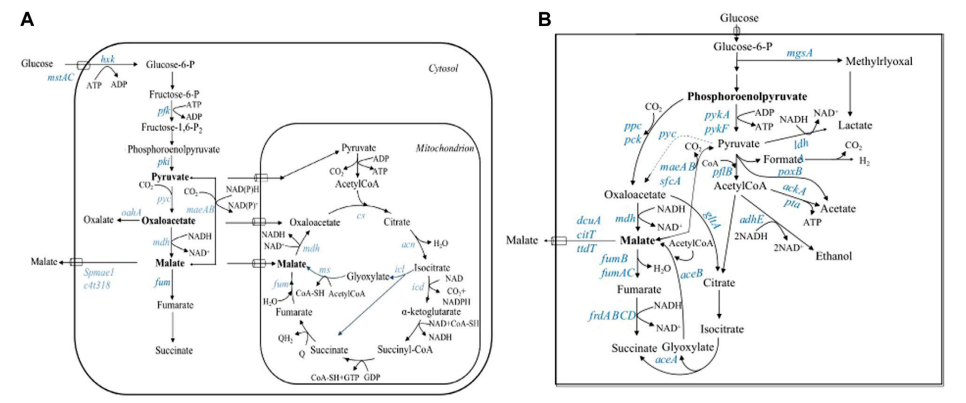
Driven by the global fossil fuel crisis and environmental issues, the development of biotechnology-based renewable fuels and chemicals has become a research hotspot. As an ideal petroleum alternative, n-butanol has attracted attention due to its superior fuel properties (high energy density, low volatility) and wide range of industrial uses. However, traditional butanol production relies on complex multi-step fermentation and high-cost cellulase production, which poses a huge challenge to achieving economic industrialization. This study combines metabolic engineering and evolutionary engineering to develop a new strategy to achieve efficient production of n-butanol by using Clostridium cellulovorans in unified biorefinery (CBP), providing a practical solution for the resource utilization of agricultural waste.

Related Services
Metabolic Engineering and Pathway Design
Bacterial Metabolic Engineering
Overview of literature research
C. Celllovorans are anaerobic bacteria with cellulose decomposition capabilities that can directly convert plant cell wall components such as cellulose and hemicellulose into organic acids. However, this strain itself does not have the ability to synthesize natural butanol and has low tolerance to butanol, so it cannot meet the needs of industrial production.
By combining metabolic engineering with adaptive laboratory evolution (ALE), this study developed an integrated strategy to simultaneously improve the n-butanol synthesis ability and tolerance of strains. In the end, the research team successfully constructed an engineering strain that could use corn cobs as the sole carbon source to produce n-butanol, and its yield was 138 times higher than that of the wild type.
Highlights
1. Metabolic engineering optimization
Researchers modified C. cellulovorans, giving it the ability to synthesize butanol. Specific methods include:
1.1 Constructing an acetyl-CoA-dependent ABE (acetone-butanol-ethanol) metabolic pathway:
The adhE1 (alcohol dehydrogenase) and ctfA-ctfB-adc (CoA transferase and acetoacetate decarboxylase) gene clusters of Clostridium acetobutylicum were introduced.
This metabolic path can convert butyric acid into butanol, while reducing the accumulation of by-products and improving the utilization efficiency of carbon flux.
1.2 Compare the effects of different gene combinations:
The study found that compared with adhE2, the expression of adhE1 increased butanol production nearly 4 times to 1.63 g/L, and significantly enhanced the activities of butyraldehyde and butanol dehydrogenase.
1.3 Control the formation of by-products:
In order to further improve the reuse rate of butyrate, the bukI gene (butyrate kinase) was introduced, but the effect was limited. Subsequently, a collaborative expression strategy of the ctfAB-adc gene cluster was adopted to improve butyrate recovery efficiency and increase butanol production by 49.3%.
2. Evolutionary engineering improves butanol tolerance
The toxicity of butanol is one of the main factors limiting fermentation performance. This study used the Adaptive Laboratory Evolution (ALE) strategy to screen highly tolerant strains by gradually increasing the butanol concentration in the medium:
- The initial strain grew poorly in a butanol environment of 3 g/L. After multiple rounds of evolution, the evolved strain (ZQW30) grew stably in a butanol environment of 12 g/L, and its tolerance was significantly improved.
- By knocking out the spo0A gene (a regulatory factor that controls spore formation), direct evolution of the strain is promoted, avoiding a false increase in tolerance to spore formation. Eventually, the modified strain showed higher survival rate and yield during the fermentation process.
3. Integrated metabolic and evolutionary engineering
The research team combined metabolic engineering with evolutionary engineering to reintroduce ABE metabolic pathway genes in evolved highly tolerant strains.
- The final engineered strain ZQW35 achieved an n-butanol yield of 3.47 g/L in the optimized 3-liter fermentation tank, a 138-fold increase compared with the wild type strain, making it the highest butanol yield in the CBP process to date. One of the reports.
- The experiment also optimized fermentation parameters, such as adopting a two-stage pH control strategy (pH 7.0 in the early stage promotes growth and pH 6.0 in the later stage promotes butanol synthesis), which increased butanol production efficiency by 31.3%.

Integrated metabolic and evolutionary engineering of Clostridium cellulovorans 743B for n-butanol production
Research significance and industrialization potential
1.Technological innovation:
This study demonstrates for the first time that C. cellulovorans can achieve efficient conversion of butyric acid to butanol.
The research demonstrates the potential of the integrated CBP process in the resource utilization of agricultural waste and provides a technical basis for low-cost biofuel and chemical production.
2.Industrialization feasibility:
- C. cellulovorans can directly use agricultural and forestry waste such as alkali-treated corn cobs as a carbon source without the need to add additional cellulase or pretreatment steps, greatly reducing production costs.
- The optimized strain has high butanol tolerance and stability, laying a foundation for large-scale industrial production.
3. Green economy benefits:
- Using corn cobs as a carbon source not only reduces the accumulation of agricultural waste, but also provides farmers with additional income channels.
- By reducing dependence on fossil fuels, it helps achieve lower carbon emissions and higher resource efficiency.
Existing challenges and future prospects
Although this study has made important breakthroughs in strain modification and fermentation process, there is still the following room for improvement:
1. Butyric acid and substrate residue issues:
- At the end of fermentation, the residual amount of butyric acid was 3.76 g/L, which was not fully utilized. By further optimizing metabolic flow (such as knocking out the buk gene), the efficiency of the conversion of butyric acid to butanol may be improved.
- The utilization efficiency of five-carbon sugars such as xylose is low, resulting in too long fermentation time. In the future, genetic engineering can enhance the expression of xylose transporters (such as XylT) and inhibit the xylose operon transcription inhibitor (XylR) to improve substrate utilization.
2. Strain stability and sustainable improvement:
- As the scale of fermentation expands, the genetic stability of the strain may become a limiting factor, and further optimization of genetic operation techniques is needed.
- Combined with multi-omic techniques (such as transcriptome and metabolome analysis), in-depth research on the molecular mechanisms of the enhanced tolerance of strains to butanol will help screen more potential transformation targets.
Conclusion
This study significantly improves the efficiency of C. The n-butanol production capabilities of cellulovorans in the CBP process demonstrate huge potential in the production of renewable fuels and chemicals. This innovation not only provides an efficient way for the resource utilization of agricultural waste, but also takes an important step towards achieving the goals of low-carbon economy and green industrialization.
Reference:
Z Wen, R Ledesma-Amaro, J Lin… – Applied and …, 2019 – Am Soc Microbiol