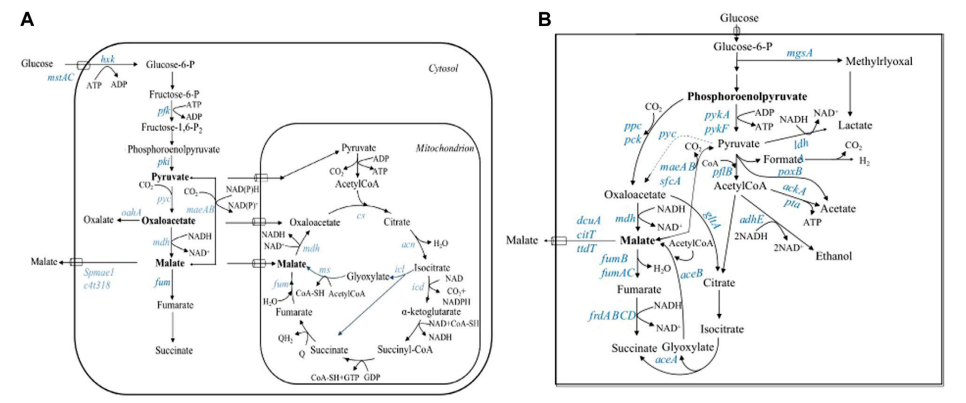
Succinic acid is a 4-carbon dicarboxylic acid. Not only a major intermediate in the TCA, it is also a major raw material in food, pharmaceutical and chemical production. Since the market for sustainable development is increasing, renewable energy for microbial fermentation of succinic acid is gaining traction in the industry. But old-school fermentation tends to be coupled with the production of byproducts (acetic acid, formic acid, lactic acid) that diminish yields and cost extra to purify.
The team’s solution to this challenge was to modify the gram-negative anaerobic bacteria Mannheimia succiniciproducens through genomics and metabolic engineering, and construct a highly productive strain of succinic acid producing bacteria that reduced by-products significantly. These results demonstrate the power of genome-scale metabolic engineering to optimise biobased chemical manufacturing.

Overview
- Increase succinic acid production;
- Eliminate the formation of by-products during the fermentation process;
- Explore the role of key metabolic enzymes in succinate synthesis.
Research methods and strategies
1 Gene knockout strategy:
- Carry out gradual knock-out of key enzyme genes for by-product production (such as ldhA, pflB, pta and ackA).
- Experiments were conducted to verify the impact of gene knockout on metabolic flow redistribution and succinate production.

2 Fermentation process optimization:
- Compare succinate production under batch culture and fed-batch culture conditions;
- Use optimized medium compositions and CO ˇ supply to enhance fermentation efficiency.
3 Analysis of key metabolic enzymes:
- To study the role of three CO ˇ-fixing enzymes (PEP carboxylase, PEP carboxykinase and malase) in succinate synthesis and analyze their contribution to the metabolic flux of C-C.
Key findings
1.Optimization of metabolic pathways and its effects
The gene knockout approach was successful as the research group had transformed M. Metabolic network of succiniciproducens:
- Inhibition of lactate production:
After knocking out the gene for lactate dehydrogenase (ldhA), lactic acid production fell sharply, succinic acid was produced at a higher rate (10.5 g/L to 12.0 g/L), and the conversion rate was 0.90 mol/mol glucose.
- Decrease formic acid and acetic acid formation:
After knocking out ldhA, the pflB (pyruvate formate lyase) gene was also knocked out and formic acid was produced at 100% without the presence of any acetate. Now the succinic acid yield was 13.4 g/L and the conversion was 0.97 mol/mol glucose.
- Whole-body blockage of acetic acid formation:
Once the genes for pta (phosphotransacetylase) and ackA (acetate kinase) were deleted, acetate production was practically eliminated. The final succinic acid production of the modified strain (LPK7) was 13.4 g/L, with acetate and lactic acid almost invisible.
- Contribution of CO fixation mechanisms
The three CO -fixing enzymes that were investigated each contribute different components to succinate synthesis:
- PEP carboxykinase (pckA):
It was discovered to be an important enzyme in C to C metabolic flux conversion. Its knockout drastically lowers the succinate (by 57%) and slows the strain’s anaerobic development.
- PEP carboxylase (ppc) and malylase (maeB):
Its knockout is less detrimental to succinic acid synthesis, but alters the distribution pattern of by-products.
- Significant advantages of fed-batch fermentation
The succinic acid production of LPK7 strain was further augmented to 52.4 g/L under fed-batch fermentation, and the productivity increased to 1.8 g/L/h. Experiments also showed that:
- By-products were not produced at all, and there was only a trace of malic acid and pyruvate;
- It climbed to 1.16 mol/mol glucose (0.76 g/g glucose), which is among the highest seen.
Industrialization potential and challenges
Technical advantages
- Efficient by-product inhibition:
Compared with other fermentation strains, the succinic acid production of LPK7 strain is hardly accompanied by the production of acetic acid, formic acid and lactic acid, greatly simplifying the downstream purification process.
- Sustainability:
Using carbon dioxide as a reaction substrate not only increases succinic acid production, but also achieves effective recycling of carbon resources.
- High conversion and production rates:
The optimized fermentation process can significantly reduce raw material consumption and production costs, providing feasibility for large-scale industrial production.
Existing challenges
- Pyruvic acid and malic acid residues:
Although major by-products were suppressed, the accumulation of pyruvate and malate limited further improvements in succinic acid production.
- Insufficient supply of reducing power:
The production of succinic acid requires a large amount of reducing power, and in the future, consideration can be given to enhancing electron supply through external hydrogen supply or optimizing the expression of reducing power-related enzymes.
- Genome stability:
The genetic stability of engineered strains in long-term fermentation or industrial applications requires further verification.