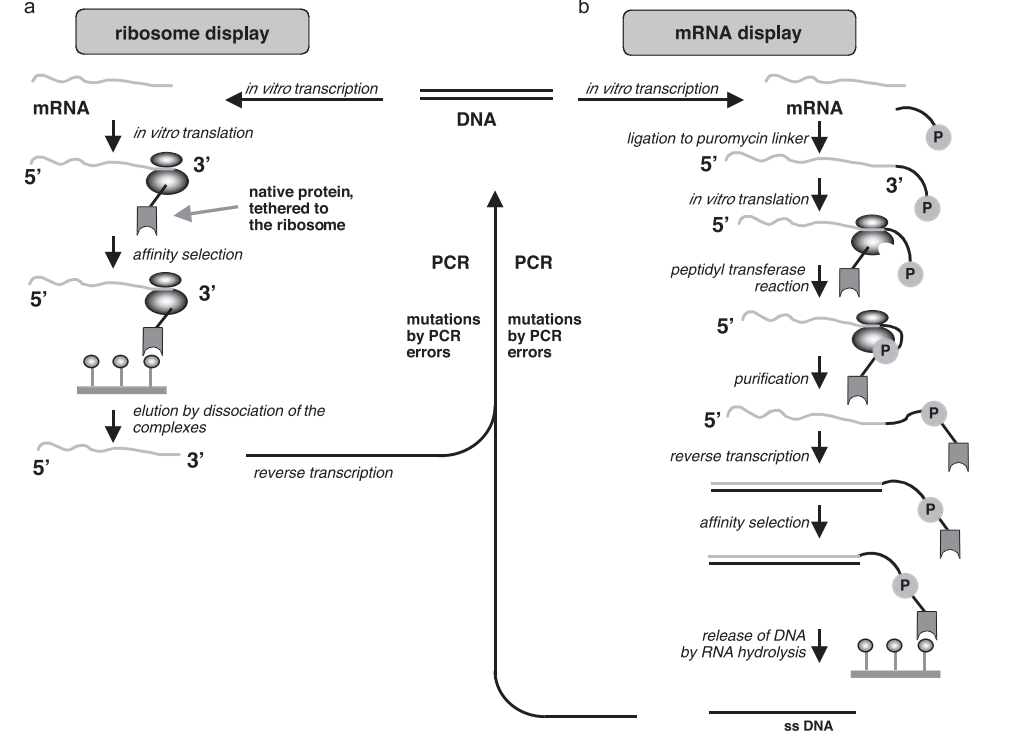
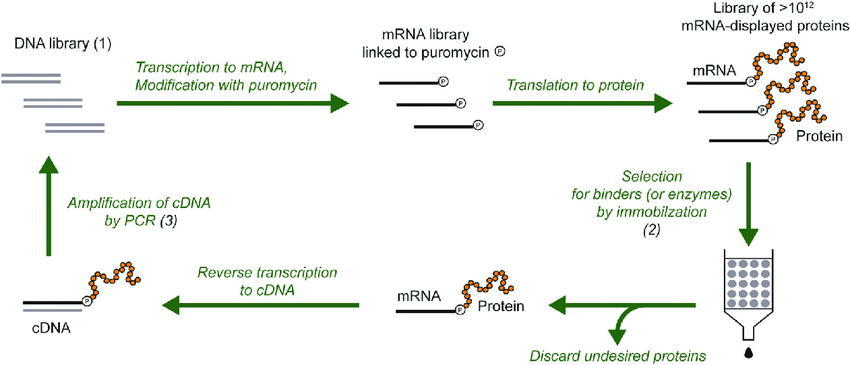
1. Introduction to Codon Optimization
Codon optimization represents an influential molecular biology method that modifies a gene’s nucleotide sequence to enhance protein production in a target organism. Scientists achieve improved translation efficiency and protein yield while preserving functional integrity when they match the gene’s codon usage to the host’s translational system. Achieving high expression levels represents a major obstacle in recombinant protein production as well as gene therapy and synthetic biology applications.
Codon optimization relies on the genetic code’s ability to encode the same amino acid with multiple codons due to its degeneracy. Distinct codon usage patterns occur in various organisms because tRNA abundance, mRNA stability, and evolutionary history affect codon preferences. The bacterium Escherichia coli (E. coli) selects codons that match well with abundant tRNAs whereas mammalian cells choose codons that support effective translation initiation and proper mRNA processing.
This article examines essential factors for codon optimization within three principal expression systems. Our research on codon optimization across E. coli, mammalian cells, and yeast systems incorporates multiple real-world case studies. Our research includes practical approaches for creating codon-optimized sequences alongside discussions of the latest developments in codon optimization technology.

Engineered polyketide synthase with applied codon optimization strategies and targeted heterologous hosts. (Schmidt, et al. 2023)
If you’re looking to apply these concepts in your research, CD Biosynsis offers a range of codon optimization services tailored to different expression systems. Their expertise can help you enhance protein production in your specific target organisms.
2. Key Considerations for Codon Optimization
Achieving effective codon optimization demands comprehensive knowledge of the host organism’s biology to balance translation efficiency with mRNA stability and protein folding. The following section outlines essential factors and tools that are important for codon optimization:
| Factor | Impact on Expression | Tools/Strategies |
| Codon Usage Bias | Determines translation speed and accuracy. | Codon usage tables, software like GeneArt, OptimumGene. |
| mRNA Stability | Affects transcript longevity and expression levels. | Avoiding destabilizing sequences (e.g., AU-rich elements). |
| GC Content | Influences DNA melting temperature and transcription. | Balancing GC percentage for host compatibility. |
| Secondary Structure | Can hinder ribosome binding and translation. | Algorithms to predict mRNA folding (e.g., mFold). |
| Regulatory Elements | Impacts transcription and translation initiation. | Incorporating Kozak sequences (mammalian) or Shine-Dalgarno sequences (bacteria). |
When scientists account for these factors they can design sequences specific to each host organism which results in stable and efficient protein production. Understanding these elements is crucial, and services that focus on codon optimization can assist in leveraging them effectively for enhanced protein expression.
3. Codon Optimization for E. coli
- coli stands as an essential organism for recombinant protein expression because of its quick growth rate together with simple genetic modification techniques and extensive genetic knowledge. E. coli’s codon usage bias significantly influences how well foreign genes express themselves.
Key Strategies for E. coli
- Avoid Rare Codons:In E. coli the tRNA population shows restricted availability for codons like AGG, AGA, and CUA. Replacing infrequently used codons like AGG with their synonymous counterparts which occur more often such as CGU for Arg results in faster translation speeds.
- Optimize mRNA Stability: Minimizing secondary structures in the mRNA 5’ UTR while removing AU-rich destabilizing elements leads to longer mRNA transcripts.
- Shine-Dalgarno Sequences: Ribosome-binding sites positioned before the start codon serve as essential elements to trigger translation. Strong binding characteristics of the Shine-Dalgarno sequence lead to higher translational efficiency.
Case Study: Enhanced RBD protein targeting SARS-CoV-2 in E. coli
To study the RBD protein targeting SARS-CoV-2, and improve its expression in E. coli BL21 (DE3). Specific steps include:
- Obtain the original RBD gene sequence from NCBI, optimize the codons to adapt to E. coli preferences, and avoid duplicate sequences and restriction cleavage sites.Services like Codon Optimization for E. coli Expression Service can simplify this process by leveraging advanced algorithms to modify the gene sequence according to E. coli’s codon usage preferences.
- The optimized gene was inserted into the pET-15b plasmid and transformed into the host strain by heat shock method.
- Verification and optimization effect: PCR confirmed the gene insertion, sequencing showed no variation in the nucleotide sequence, and electrophoresis analyzed the stability of the plasmid.
- Key optimization indicators: CAI (Codon Adaptation Index) increased from 0.72 to 0.96, indicating that gene translation efficiency has been significantly improved.
Comparison of data before and after optimization
| parameters | before optimization | optimized |
| Cai values | 0.72 | 0.96 |
| plasmid stability | There are potential instability factors | Electrophoresis confirmed that the plasmid size was consistent |
| sequencing results | – | No nucleotide/amino acid mutations |
| Expression system adaptability | low (non-preferred codon) | High (matches host preference) |
4. Codon Optimization for Mammalian Cells
HEK293 and CHO cells serve as vital mammalian expression systems for generating complex proteins with accurate post-translational modifications. The codon usage and regulatory requirements of mammalian systems present substantial differences compared to prokaryotic systems.
Key Strategies for Mammalian Cells
- Kozak Consensus Sequence:The translation initiation process improves when the Kozak sequence (GCCGCCATGG) is positioned around the start codon.
- Avoid Cryptic Splice Sites:The optimized sequence should prevent the accidental formation of splice donor or acceptor sites that lead to mRNA misprocessing.
- mRNA Stability Elements: The addition of stabilizing sequences such as the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) together with the elimination of AU-rich destabilizing motifs improves mRNA stability.
Case Study: Luciferase Production
The research team codon-optimized the bacterial luciferase gene (luxA/luxB) to improve its expression in mammalian cells (HEK293). Three stable cell lines were constructed: wild-type luxA/luxB (WTA/WTB), optimized luxA+ wild luxB (COA/WTB), and optimized luxA/luxB (COA/COB). The results showed that codon optimization significantly increased LuxA protein levels and bioluminescence intensity, while mRNA levels were not affected.
Data comparison table
| parameters | Wild type (WTA/WTB) | Optimize luxA+ wild luxB (COA/WTB) | Double optimization (COA/COB) |
| mRNA levels | reference value | no significant change | no significant change |
| LuxA protein expression | low | significant increase | highest |
| Bioluminescence intensity (RLU/mg) | 5×10⁵ | 2.9×10 (6 times increase) | 2.7×10 (54 times increase) |
For those working with mammalian cells, Mammalian Codon Optimization Service can be a valuable resource. It offers customized strategies to optimize codon usage in mammalian cells, leading to enhanced protein production.
5. Codon Optimization for Yeast
Saccharomyces cerevisiae and Pichia pastoris combine prokaryotic simplicity with eukaryotic protein processing capabilities. To optimize codons for yeast expression systems scientists must overcome specific challenges unique to this organism.
Key Strategies for Yeast
- Codon Bias Adjustment:In yeast high expression genes demonstrate a preference for codons that terminate with either A or T nucleotides. An example of codon optimization in yeast involves selecting UUA as the preferred codon for Leu rather than the typical CUG.
- GC Content Balance: Ensuring the GC content remains within 35-45% in S. cerevisiae prevents transcriptional problems.
- Post-Translational Modifications:Sequence optimization needs to be tailored to enable glycosylation because different yeast species exhibit various glycosylation patterns.
Case Study: Recombinant Enzyme Production in Yeast
Researchers analyzed expression results by separately introducing the natural gene and the codon-optimized gene (ROL and phyA) into yeast cells. The study results demonstrated that codon optimization led to significant improvements in both the yield and enzyme activity of the recombinant protein. Following 96 hours of induction the optimized ROL gene showed 2.7 mg/mL protein content and 220.0 U/mL lipase activity which surpassed the original gene values of 0.4 mg/mL and 118.5 U/mL. Optimization of the phyA gene resulted in protein content rising to 2.2 mg/mL and phytase activity reaching 122 U/mL compared to original levels of 0.35 mg/mL and 25.6 U/mL.
Data comparison table
| Gene | parameters | before optimization | optimized | Increase multiple |
| ROL | Protein content (mg/mL) | 0.4 | 2.7 | 6.75x |
| Lipase activity (U/mL) | 118.5 | 220.0 | 1.86x | |
| phyA | Protein content (mg/mL) | 0.35 | 2.2 | 6.29x |
| Phytase activity (U/mL) | 25.6 | 122 | 4.77x |
If you’re conducting research on yeast protein expression, Yeast Codon Optimization Service can help you navigate the unique challenges of yeast codon usage and enhance your protein production.
6. Designing a Codon-Optimized Sequence: A Step-by-Step Approach
- Identify the Host Organism: Analyze the codon usage bias of the target expression system using resources like the Codon Usage Database.
- Select Optimization Software: Tools like GeneArt, OptimumGene, or JCat generate synthetic sequences tailored to the host’s preferences.
- Adjust for mRNA Stability: Use algorithms like RNAfold to minimize secondary structures in the 5’ UTR.
- Incorporate Regulatory Elements: Add Kozak sequences (mammalian) or Shine-Dalgarno sequences (bacteria) as needed.
- Validate Experimentally: Test the optimized sequence in the host organism and refine based on expression results.
These steps form the basis of effective codon optimization, and services that offer comprehensive Codon Optimization for Protein Expression can guide you through each stage, ensuring optimal results.
7. Challenges and Future Trends in Codon Optimization
Despite its effectiveness, codon optimization faces challenges. Excessive focus on translation speed during optimization causes protein misfolding because ribosomes move along the mRNA too rapidly for proper protein folding to occur. Current knowledge about how neighboring codons affect translation efficiency through context-dependent effects is limited.
Upcoming developments in codon optimization will probably use machine learning techniques to establish optimal sequences from extensive expression data sets. Artificial Intelligence tools can evaluate protein structure combined with mRNA stability and host-specific translational dynamics to achieve holistic optimization.
8. Conclusion
Protein expression across different host organisms can be maximized through the application of codon optimization techniques. Researchers who customize DNA sequences for E. coli, mammalian cells, and yeast can surpass translation rate limitations and obtain enhanced production rates along with superior protein quality and stable performance.
Our case studies demonstrate how proper optimization techniques such as bacterial rare codon replacement, mammalian Kozak sequence enhancement or yeast GC content balancing lead to significant improvements in recombinant protein production. The development of computational technology alongside synthetic biology advancements indicates that codon optimization will deliver increasingly accurate and efficient solutions within biotechnology and medical fields.
Related Services
Beyond codon optimization, CD Biosynsis offers a variety of other services related to protein expression:
- Recombinant Protein Expression Service: This comprehensive service covers all steps from gene expression and purification to protein expression and purification, with options for multiple expression platforms such as bacterial, yeast, insect cell, and mammalian cell platforms.
- Protein Expression in E. coli Service: Specializes in recombinant protein expression through E. coli, providing services like gene synthesis, vector construction, and protein purification.
- Protein Expression in Yeast: Dedicated to yeast protein expression, offering a complete service spectrum from gene cloning to protein purification. It also includes options for different yeast strains, such as Saccharomyces cerevisiae and Pichia pastoris.
- Custom Pichia pastoris Protein Expression Service: Tailor-made solutions for recombinant protein expression using Pichia pastoris, an efficient host for industrial-scale protein production.
- Custom Saccharomyces cerevisiae Protein Expression Service: Specialized in producing recombinant proteins in Saccharomyces cerevisiae, providing comprehensive support from project design to protein purification.
- Protein Expression in Insect Cells: A method for producing recombinant proteins using insect cells, which can perform proper protein folding and post-translational modifications.
- Protein Expression in Mammalian Cells: Focuses on producing proteins using mammalian cell lines like CHO and HEK cells, which can correctly fold and modify proteins.
- Custom CHO Cell Protein Expression Service: Offers specialized solutions for producing recombinant proteins in CHO cells, known for their high protein yield and human-like glycosylation.
- Custom HEK Cell Protein Expression Service: Utilizes HEK cells to efficiently produce recombinant proteins, suitable for diverse biotechnological and therapeutic applications.
- Multigene Expression Systems Services: Provide advanced solutions for simultaneously expressing multiple genes within a single host, facilitating complex genetic studies and synthetic biology applications.
Reference
Purnamasari, Elly Widyarni Eka, et al. “Optimasi Kodon dan Konstruksi Plasmid Rekombinan Protein RBD SARS-CoV-2 pada E. coli BL21 (DE3).” Journal of Health (JoH) 11.1 (2024): 043-051.
Yang, Jiang-Ke, et al. “A simple and accurate two-step long DNA sequences synthesis strategy to improve heterologous gene expression in Pichia.” PLoS One 7.5 (2012): e36607.
Schmidt, Matthias, et al. “Maximizing heterologous expression of engineered type I polyketide synthases: Investigating codon optimization strategies.” ACS Synthetic Biology 12.11 (2023): 3366-3380.